Mole Conversions Tutorial 1mole =602,214,150,000,000,000,000,000 Avogadro's NumberThe number of carbon-12 atoms in 12 grams of unbound carbon in the ground state. Avogadro's number, the number of particles in a mole, can be experimentally determined by first 'counting' the number of atoms in a smaller space and then scaling up to find the number of particles that would have a mass equal to the atomic or molecular mass in grams. Here is some real data from which Avogadro's number can be determined.X-Ray diffraction studies show that gold consists of a repeating atomic arrangement where the repeating unit (called a cell unit) is a cube containing 4 gold atoms. Each side of the cube has a length of 4.08x10-8 cm. The density of gold is 19.3 g / cm3 and its atomic mass is 197.
The History of the Term'Mole' The Avogadro constant is named after the early nineteenth century Italian scientist Amedeo Avogadro, who is credited (1811) with being the first to realize that the volume of a gas (strictly, of an ideal gas) is proportional to the number of atoms or molecules. The French chemist Jean Baptiste Perrin in 1909 proposed naming the constant in honor of Avogadro. American chemistry textbooks picked it up in the 1930's followed by high school textbooks starting in the 1950s. The unit 'mole' was introduced into chemistry around 1900 by Ostwald, and he originally defined this unit in terms of gram. Gram is a unit of mass; but what is the mole a unit of? Ostwald did not say;3 however, several years later, he did make it clear that the concept of mole should be linked to the ideal gas. 4 3'...the molecular weight of a substance, expressed in grams, shall henceforth be called mole [. . . das in Grammen augedruckte [. . .] Molekulargewicht eines Stoffes soll fortan ein Mol heissen]' Ref. 7). 4 'That amount of any gas that occupies a volume of 22414 mL in normal conditions is called one mole [eine solche Menge irgendeines Gases, welche das Volum von 22412 ccm im Normalzustand einnimt nennt man ein Mol]' References Ref. 8. Ostwald, W. Grundriss der allgemeinen Chemie, 5th ed.; Dresden: Steinkopff, 1917, p. 44 From-http://dbhs.wvusd.k12.ca.us/webdocs/Mole/Origin-of-Mole.html Real World Moles (wiki)
Mole Conversions Tutorial Chemical Demonstration Videos |
- The Mole In Chemistry
- Chemistry Mole And Avogadro's Number
- Avogadro's Number
- How To Calculate Moles In Chemistry
There are about 20 species of fuzzy moles (the kind that crawl around on the ground), and one kind of mole that might appear on your face, but today is a day for celebrating a different kind of mole. Today’s mole is a unit — one invented by a man named Avogadro.
Really, a mole is just a group of objects. You can think of “a mole” the same way you think of “a dozen.” You’re probably familiar with a dozen eggs, or chickens or planets. Moles are no different. You can have a mole of molecules or people or cheeseburgers. But there are a lot more than twelve things in a mole — there are 6.02 x 1023. That’s 602,000,000,000,000,000,000,000 things. Because the mole contains so many units, they’re most often used in chemistry is a way of measuring really really small things like atoms or molecules.
. The molar mass of a compound is found by adding together the molar masses of all of its elements, taking into account the number of moles of each element present. Recall: atomic mass = molar mass if there is 1 mole of substance. e.g. The mole is equal to Avogadro's numb. The mole is a unit of measure for the amount of a chemical substance. The mole is an important concept for talking about a very large number of things — 6.02 x 1023 of them to be exact. This module shows how the mole, known as Avogadro’s number, is key to calculating quantities of atoms and molecules. It describes 19th-century developments that led to the concept of the mole, Topics include atomic weight, molecular weight, and molar mass. The mole (abbreviated mol) is the SI measure of quantity of a “chemical entity,” such as atoms, electrons, or protons. It is defined as the amount of a substance that contains as many particles as there are atoms in 12 grams of pure carbon-12. So, 1 mol contains 6.022×10 23 elementary entities of the substance.
Chemistry uses a unit called mole. A mole (mol) is a number of things equal to the number of atoms in exactly 12 g of carbon-12. Experimental measurements have determined that this number is very large: 1 mol = 6.02214179 × 1023 things.
So a mole of water is 6.02 x 1023 molecules of water, which works out to be about 18 grams, or 18 mL. A mole of aluminum is about 26 grams. But to really appreciate how many molecules are in a mole it helps to think about things we can see. To do that, let’s try these comparisons from Daniel Dulek’s lesson about how big a mole is.
A way to think about the relative size of a mole?
If you had a mole of doughnuts, they would cover the entire Earth in a doughnut-layer five miles deep.
If you had a mole of basketballs, you could create a new planet the size of the Earth!
The Mole In Chemistry
If you received a mole of pennies on the day you were born, and spent a million dollars a second until you died at 100, you’d still have over 99.99% of your money in the bank.
One mole of red blood cells is more red blood cells than exist in every human on earth right now. A mole of cereal boxes stacked end to end would reach from the Sun to Pluto 7.5 million times. A mole of turkeys could form sixteen earths.
Chemistry Mole And Avogadro's Number
Okay, so now that we know why a mole has 6.02 x 1023 things in it, what can we do with that information? Moles in chemistry are far more useful than moles in the ground, or on your face. They factor into all kinds of equations and important concepts in chemistry. Chemists think of moles or atoms like you and I think of a dozen eggs – we add them to recipes, order them from the store and calculate what we need based on how many we have.
Avogadro's Number
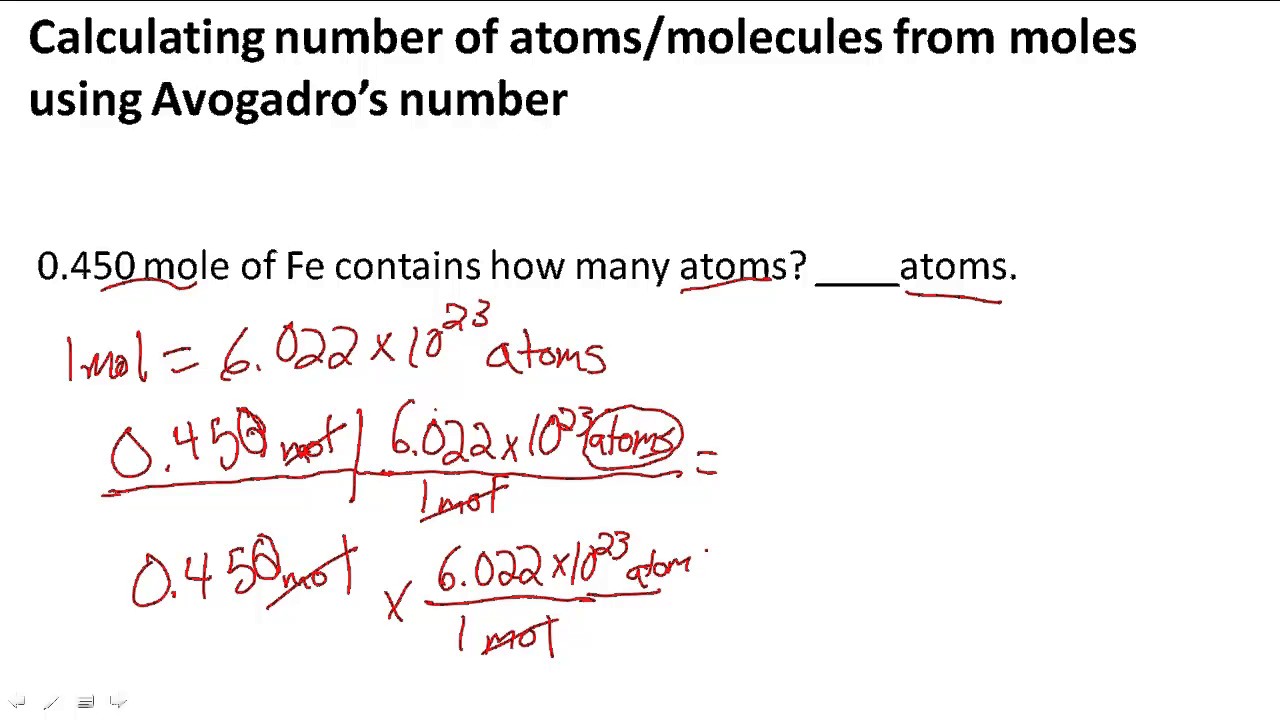
How To Calculate Moles In Chemistry
What would a mole of fuzzy moles look like? Well, the pile of animals would weigh a little over half of the mass of our moon. Thankfully, there’s no chemistry equation in the world that calls for that many mammals. But if there was, you’d now know just how many mammals that would be.
